Tutorial
This icon indicates there is a poll question. Click it when you see it to interact with your peers.
Tutorial
This icon indicates there is a poll question. Click it when you see it to interact with your peers.
Tutorial
This icon indicates there is a poll question. Click it when you see it to interact with your peers.
touchIN CONVERSATION
 A relaxed discussion between two faculty focussed on real world clinical issues. Useful tips below will show how to navigate the activity. Join the conversation.
Close
A relaxed discussion between two faculty focussed on real world clinical issues. Useful tips below will show how to navigate the activity. Join the conversation.
Close
 A relaxed discussion between two faculty focussed on real world clinical issues. Useful tips below will show how to navigate the activity. Join the conversation.
Close
A relaxed discussion between two faculty focussed on real world clinical issues. Useful tips below will show how to navigate the activity. Join the conversation.
Close
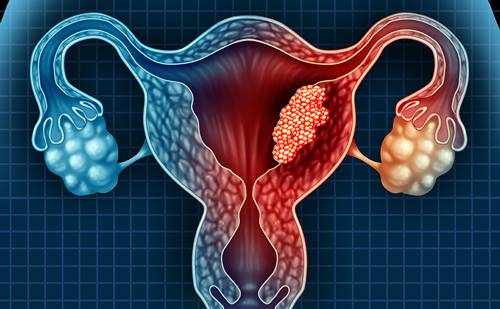
The evolving standard of care for advanced/recurrent endometrial cancer: A practice-focused discussion of immunotherapy
Learning Objectives
After watching this activity, participants should be better able to:
- Explain the impact of approved immunotherapies on standard of care for advanced/recurrent EC in the first and later lines
- Summarize key practical considerations for treating and managing patients with advanced/recurrent EC using ICI-based therapeutic strategies
- Interpret the potential role of emerging data in further influencing the treatment paradigm for advanced/recurrent EC in first- and later-line settings
Overview
In this activity, two expert gynaecologic oncology specialists discuss the evolving role of immunotherapy in the treatment of advanced/recurrent endometrial cancer (EC). They explore how immune checkpoint inhibitors (ICIs) are reshaping standards of care across lines of therapy, highlight key practical considerations for optimizing patient selection and management, and examine emerging data that may influence future treatment paradigms. read more
Target Audience
Oncologists, including gynaecologic oncology specialists, and pathologists involved in the management of advanced/recurrent EC.
EBAC® Accreditation
touchIME is an EBAC® accredited provider since 2023.
This programme is accredited by the European Board for Accreditation of Continuing Education for Health Professionals (EBAC®) for 53 minutes of effective education time.
The Accreditation Council for Continuing Medical Education (ACCME) and the Royal College of Physicians and Surgeons of Canada hold an agreement on mutual recognition on substantive equivalency of accreditation systems with EBAC®.
Through an agreement between the European Board for Accreditation of Continuing Education for Health Professionals and the American Medical Association (AMA), physicians may convert EBAC® CE credits to AMA PRA Category 1 CreditsTM. Information on the process to convert EBAC® credit to AMA credit can be found on the AMA website. Other healthcare professionals may obtain from the AMA a certificate of having participated in an activity eligible for conversion of credit to AMA PRA Category 1 CreditTM.
Faculty Disclosure Statement / Conflict of Interest Policy
In compliance with EBAC® guidelines, all speakers/chairpersons participating in this programme have disclosed or indicated potential conflicts of interest, which might cause a bias in the presentations. The Organizing Committee/Course Director is responsible for ensuring that all potential conflicts of interest relevant to the event have been mitigated and declared to the audience prior to the CME activities.
Faculty
Dr Alexandra Leary discloses: Advisory board or panel fees from AbbVie, AstraZeneca, Daiichi Sankyo, GSK, ImmunoGen, MSD, pharma& and Zentalis. Consultancy fees from Owkin and Pegascy. Grants/research support from AstraZeneca and Zentalis.
Dr Juan Francisco Grau discloses: Advisory board or panel fees from AstraZeneca. Speaker Bureau fees from AstraZeneca, GSK and pharma&.
Touch Medical Contributors
Christina Mackins-Crabtree has no financial interests/relationships or affiliations in relation to this activity.
Requirements for Successful Completion
Certificates of Completion may be awarded upon successful completion of the post-test and evaluation form. If you have completed one hour or more of effective education through EBAC® accredited CE activities, please contact us at accreditation@touchime.org to receive your EBAC® CE credit certificate. EBAC® grants 1 CE credit for every hour of education completed.
Date of original release: 10 April 2025. Date credits expire: 10 April 2027.
Time to complete: 53 minutes
If you have any questions regarding the EBAC® credits, please contact accreditation@touchime.org
To obtain the CE/CME credit(s) from this activity, please complete this post-activity test.
Claim CreditYou may also be interested in...

REGISTER NOW FOR FREE ACCESS TO
- 1000+ topical and insightful peer-reviewed journal articles
- 100+ hours of bite-sized congress highlights
- 10 major therapy areas packed with the latest scientific advances
- 150+ specialties offering learn-on-the-go medical education
- + Concise email updates and newsletters so you never miss out

Log into your Touch Account
Earn and track your CME credits on the go, save articles for later, and follow the latest congress coverage.
Sign up with an Email
Or use a .
This Functionality is for
Members Only
Explore the latest in medical education and stay current in your field. Create a free account to track your learning.