touchCONGRESS Optimizing frontline immunotherapy for advanced NSCLC
Watch this two-part activity exploring recent developments on the use of frontline immunotherapeutic strategies in patients with advanced non-small cell lung cancer (NSCLC). Filmed following the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting and European Society for Medical Oncology (ESMO) Congress 2021.
Part 1: Watch thoracic oncology expert Prof. Edward Garon review key data from the 2021 ASCO Annual Meeting and ESMO Congress 2021 Watch Now
Part 2: Choose from leading experts who discuss what these data may mean for global and regional practice Select An Interview
Introduction
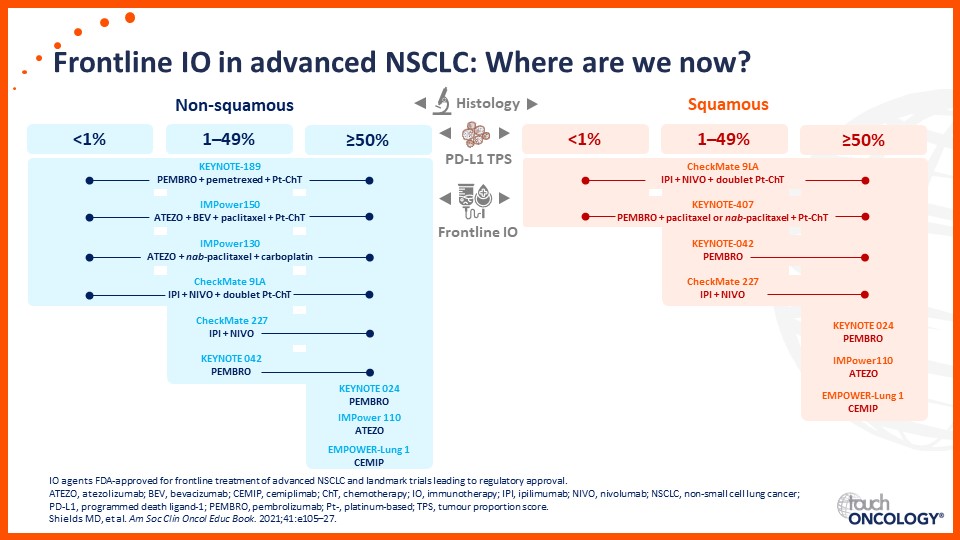
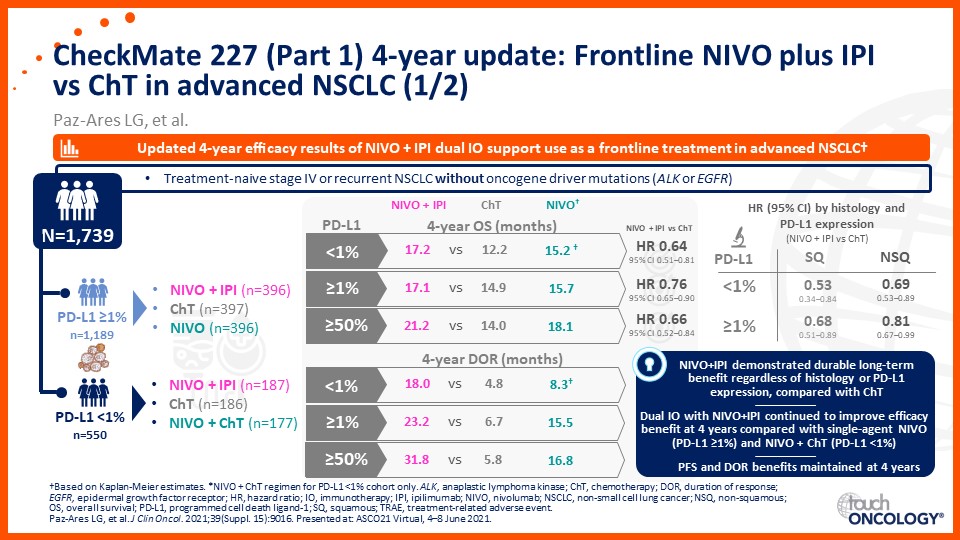
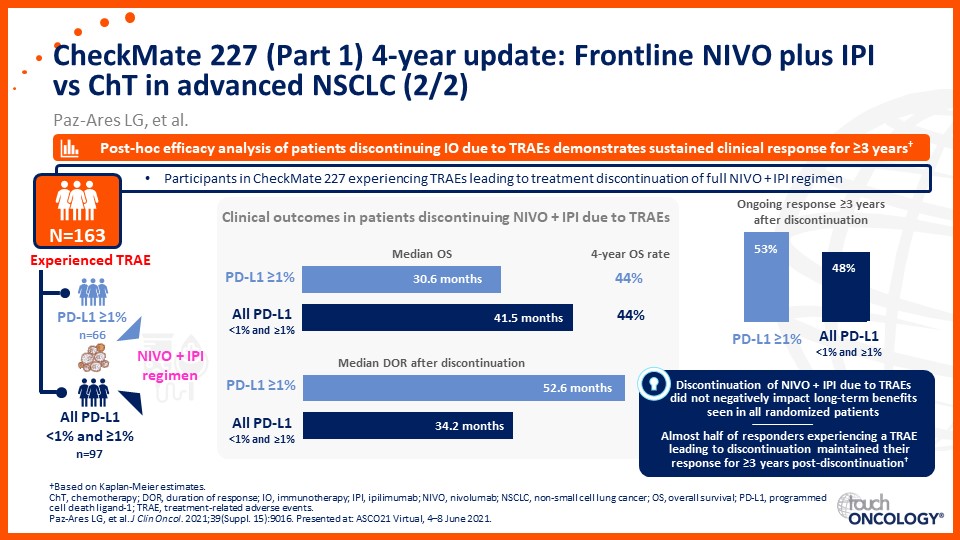
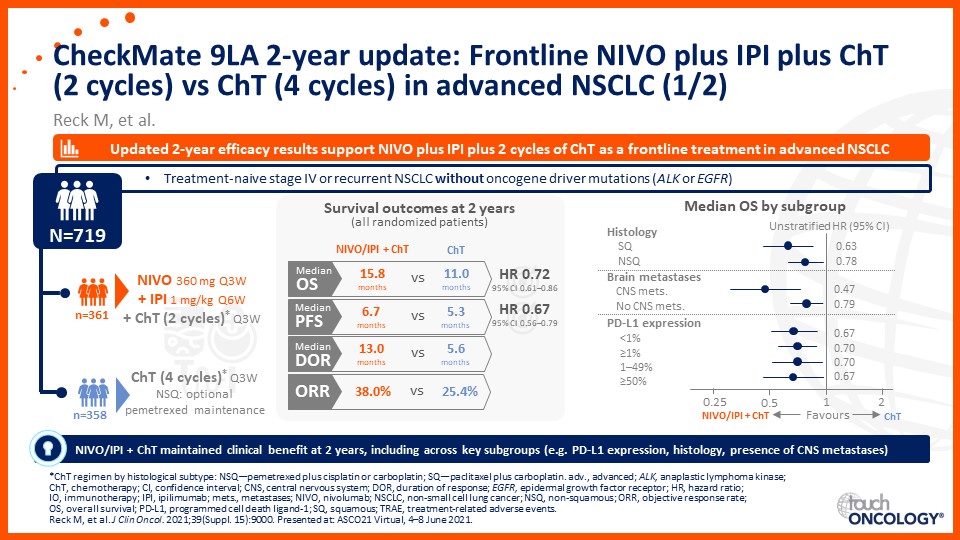
Update on immunotherapy in the frontline setting in advanced NSCLC
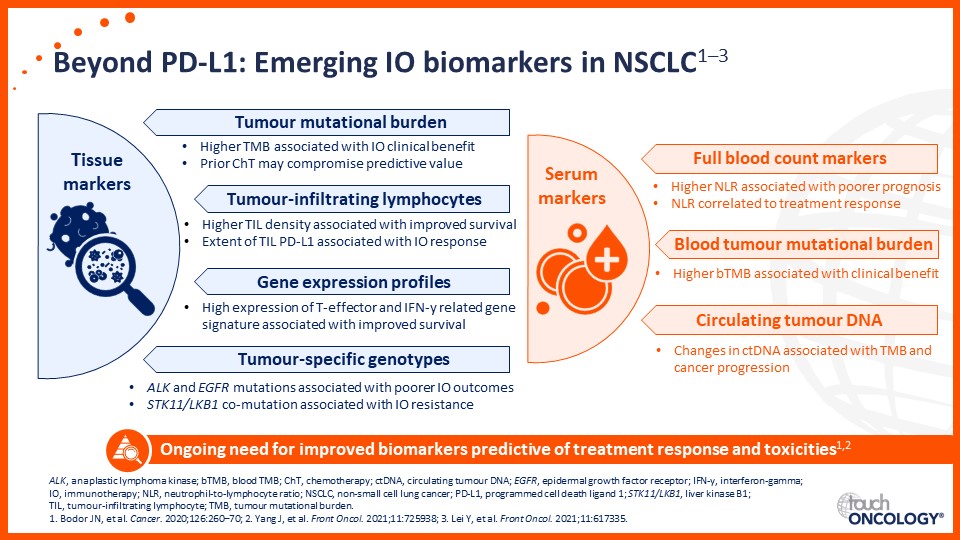
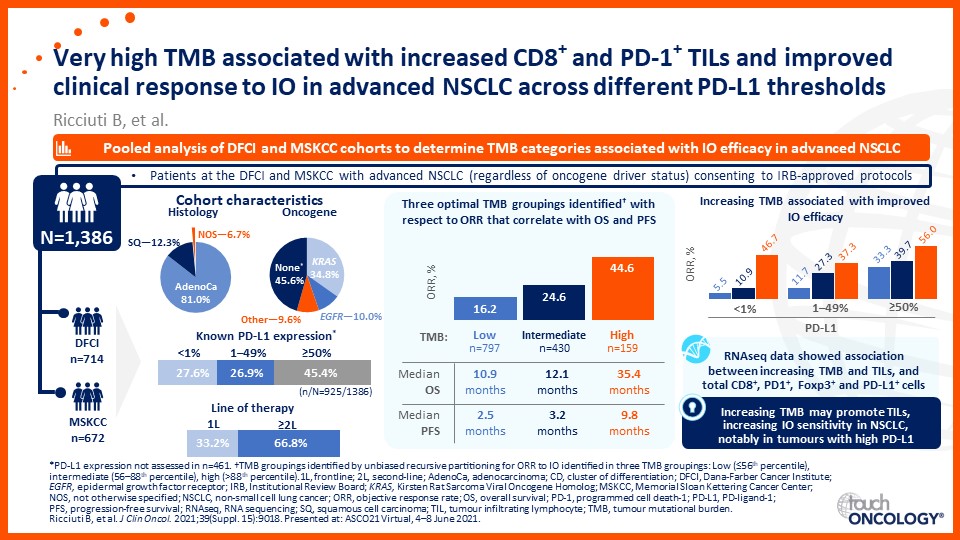
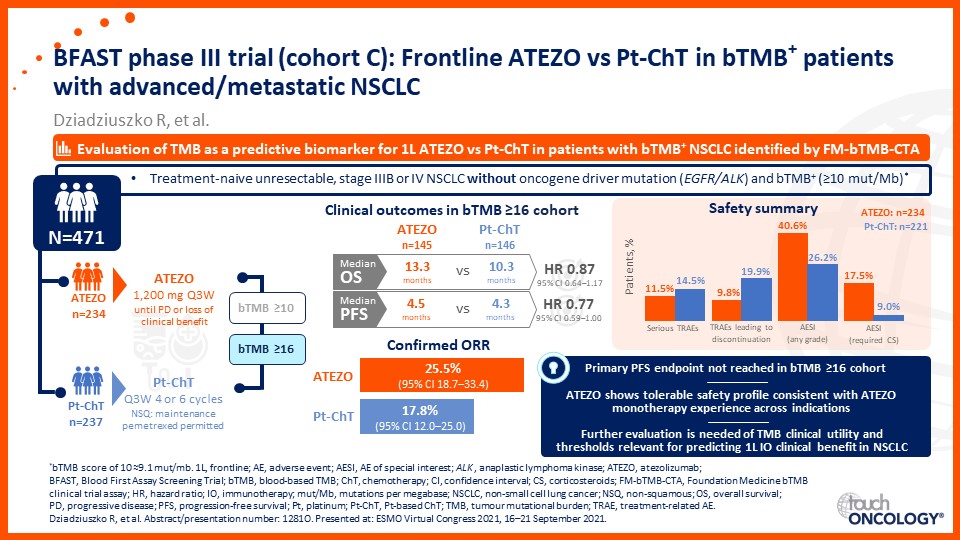
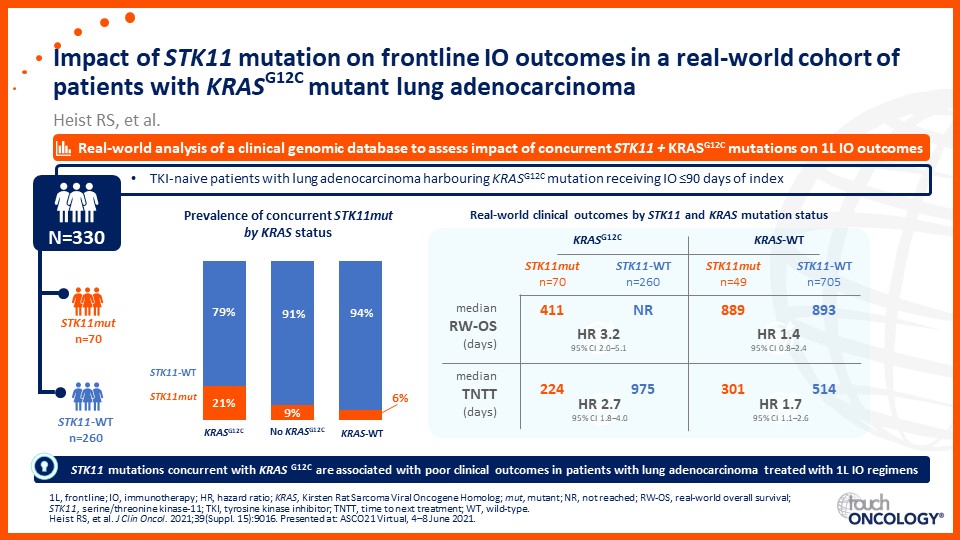
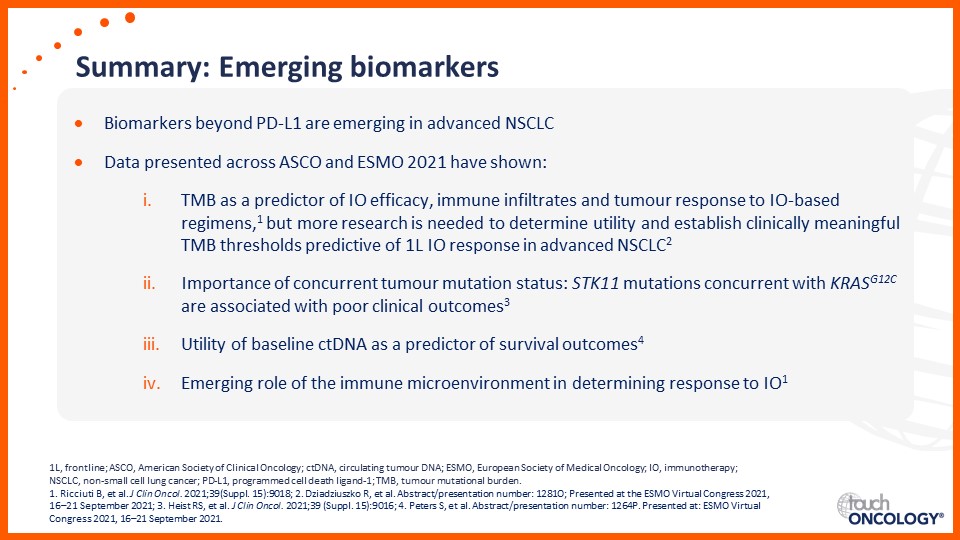
Emerging biomarkers guiding immunotherapy treatment decisions in advanced NSCLC
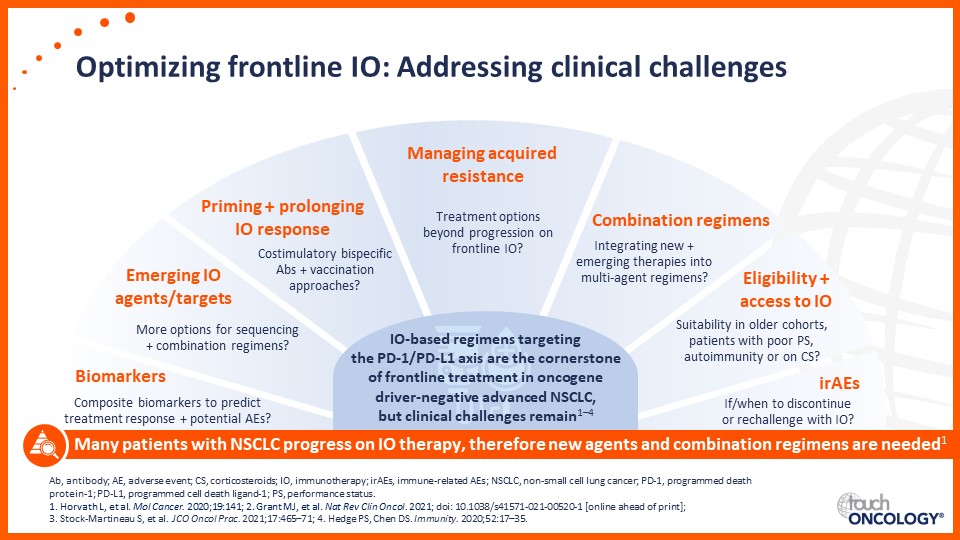
Optimizing frontline immunotherapy in the management of advanced NSCLC
Overview
Watch Prof. Edward Garon contextualize potential implications of the latest data on frontline immunotherapy in advanced non-small cell lung cancer (NSCLC), presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting and the European Society for Medical Oncology (ESMO) Congress 2021. During the presentation, Dr Garon considers:
- The latest updates on frontline immunotherapy in advanced NSCLC
- Emerging biomarkers guiding immunotherapy treatment decisions for patients with advanced NSCLC in the frontline setting
- Approaches to optimize frontline immunotherapy in the clinical management of advanced NSCLC
Edward Garon is the Director of the Thoracic Oncology Program at the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles (UCLA). He earned a bachelor’s degree in biology at the Massachusetts Institute of Technology. His medical doctorate was from Washington University in St. Louis. He performed his internship and residency at the University of Chicago. After a chief residency at Cook County Hospital in Chicago, he was a fellow in haematology and oncology at UCLA. He has remained at UCLA ever since and is currently Professor of Medicine. He also received a master’s degree in clinical investigation from UCLA.
He has been the principal investigator of peer-reviewed grants from various funding organizations, including the National Cancer Institute. His focus is on clinical research and biomarker development. He has served as the principal investigator on national and international phase I, II and III clinical trials, including trials that have led to the approval of multiple drugs and a companion diagnostic.
Disclosures
Prof. Edward Garon discloses: Advisory board or panel fees from ABL Bio, Boehringer-Ingelheim, Bristol Myers Squibb, Dracen Pharmaceuticals, EMD Serono, Eisai, GlaxoSmithKline, Merck, Natera, Novartis, Regeneron, Sanofi, Shionogi and Xilio Therapeutics; and Grants/research support from AstraZeneca, Bristol Myers Squibb, Dynavax Technologies, Lilly, EMD Serono, Genentech, Lovance Biotherapeutics, Merck, Mirati Therapeutics, Neon Healthcare and Novartis.
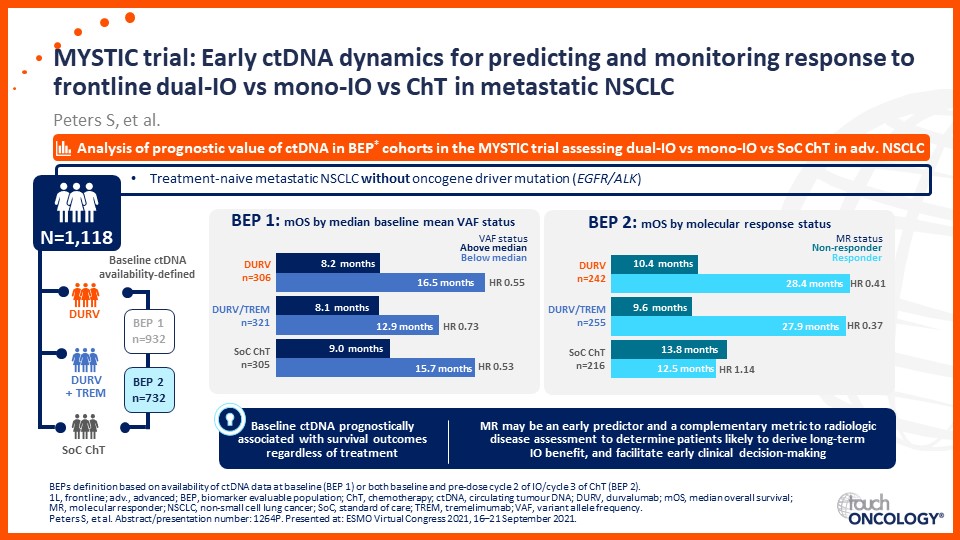
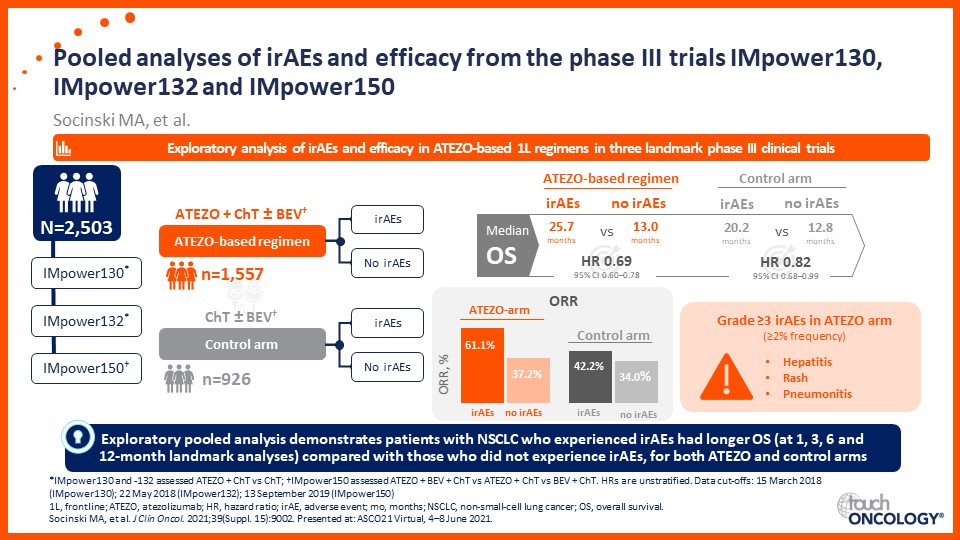
Prof. Edward Garon considers the latest data on frontline immunotherapy in advanced NSCLC, presented at the 2021 ASCO Annual Meeting and ESMO Congress 2021.
Prof. Anne-Marie Dingemans considers the latest data on frontline immunotherapy in advanced NSCLC, presented at the 2021 ASCO Annual Meeting and ESMO Congress 2021.
Dr Raffaele Califano considers the latest data on frontline immunotherapy in advanced NSCLC, presented at the 2021 ASCO Annual Meeting and ESMO Congress 2021.
Please Select A Video:
Overview & Learning Objectives
Overview
Watch our expert faculty contextualize potential implications of the latest data on frontline immunotherapy in advanced non-small cell lung cancer (NSCLC), presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting and the European Society for Medical Oncology (ESMO) Congress 2021. During the presentation, they consider:
- The latest updates on frontline immunotherapy in advanced NSCLC
- Emerging biomarkers guiding immunotherapy treatment decisions for patients with advanced NSCLC in the frontline setting
- Approaches to optimize frontline immunotherapy in the clinical management of advanced NSCLC
This activity is jointly provided by USF Health and touchIME.
Target Audience
This activity has been designed to meet the educational needs of practising oncology and respiratory specialists, with a focus on lung cancer specialists and pulmonologists involved in the management of NSCLC.
Disclosures
All individuals in a position to influence content have disclosed to USF Health any financial relationship with an ineligible organization. USF Health has reviewed and mitigated all relevant financial relationships related to the content of the activity. The relevant relationships are listed below. All individuals not listed have no relevant financial relationships.
Faculty
Prof. Edward Garon discloses: Advisory board or panel fees from ABL Bio, Boehringer-Ingelheim, Bristol Myers Squibb, Dracen Pharmaceuticals, EMD Serono, Eisai, GlaxoSmithKline, Merck, Natera, Novartis, Regeneron, Sanofi, Shionogi and Xilio Therapeutics; and Grants/research support from AstraZeneca, Bristol Myers Squibb, Dynavax Technologies, Lilly, EMD Serono, Genentech, Lovance Biotherapeutics, Merck, Mirati Therapeutics, Neon Healthcare and Novartis.
Prof. Anne-Marie Dingemans discloses: Advisory board or panel fees from Amgen, Bayer, Boehringer Ingelheim, Pharmamar, Roche and Sanofi; and Honoraria from AstraZeneca, Lilly, Janssen, Pfizer and Takeda.
Dr Raffaele Califano discloses: Advisory board or panel fees from: AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme and Roche; Consultancy fees from AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme and Roche; and Speaker bureau fees from AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme and Roche.
Content Reviewer
Angela Massey Hill, PharmD, CPh, RPh has no financial interests/relationships or affiliations in relation to this activity.
Touch Medical Director
Christina Mackins-Crabtree has no financial interests/relationships or affiliations in relation to this activity.
USF Health Office of Continuing Professional Development and touchIME staff have no financial interests/relationships or affiliations in relation to this activity.
Requirements for Successful Completion
In order to receive credit for this activity, participants must review the content and complete the post-test and evaluation form. Statements of credit are awarded upon successful completion of the post-test and evaluation form.
If you have questions regarding credit please contact cpdsupport@usf.edu
Accreditations
Physicians
This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through a joint providership of USF Health and touchIME. USF Health is accredited by the ACCME to provide continuing medical education for physicians.
USF Health designates this enduring material for a maximum of 1.5 AMA PRA Category 1 CreditsTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
The European Union of Medical Specialists (UEMS) – European Accreditation Council for Continuing Medical Education (EACCME) has an agreement of mutual recognition of continuing medical education (CME) credit with the American Medical Association (AMA). European physicians interested in converting AMA PRA Category 1 CreditTM into European CME credit (ECMEC) should contact the UEMS (www.uems.eu)
Advanced Practice Providers
Physician Assistants may claim a maximum of 1.5 Category 1 credits for completing this activity. NCCPA accepts AMA PRA Category 1 CreditTM from organizations accredited by ACCME or a recognized state medical society.
The AANPCP accepts certificates of participation for educational activities approved for AMA PRA Category 1 CreditTM by ACCME-accredited providers. APRNs who participate will receive a certificate of completion commensurate with the extent of their participation.
Date of original release: 07 October 2021. Date credit expire: 07 October 2022.
If you have any questions regarding credit please contact cpdsupport@usf.edu
Learning Objectives
After watching this activity, participants should be better able to:
- Summarize recent data evaluating frontline single-agent immunotherapy or combination strategies for advanced NSCLC
- Assess potential biomarkers that may be used to identify patients with advanced NSCLC who may benefit from frontline immunotherapy
- Outline effective management strategies using frontline immunotherapeutic strategies for patients with advanced NSCLC

Register to touchONCOLOGY for FREE
- Peer-reviewed journals and expert opinions
- Interactive CME and e-learning modules
- Video conference highlights